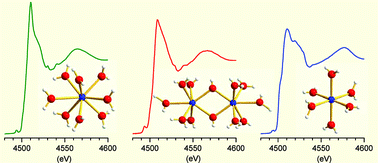
The structures of the hydrated scandium(III) ion and of the hydrated dimeric hydrolysis complex, [Sc2(µ-OH)2]4+, in acidic aqueous solutions have been characterized by X-ray absorption fine structure (XAFS) and large-angle X-ray scattering (LAXS) methods. Comparisons with crystalline reference compounds containing hydrated scandium(III) ions in well characterized six-, seven- and eight-coordinated polyhedra have been used to evaluate the coordination numbers and configurations in aqueous solution. In strongly acidic aqueous solution the structure of the hydrated scandium(III) ion is found to be similar to that of the eight-coordinated scandium(III) ion with distorted bicapped trigonal prismatic coordinating geometry in the crystalline [Sc(H2O)8.0](CF3SO3)3 compound. The EXAFS data reveal for the solution, as for the solid, a mean Sc–O bond distance of 2.17(1) Å to six strongly bound prism water molecules, 2.32(4) Å to one capping position, with possibly another capping position at about 2.5 Å. The LAXS study supports this structural model and shows furthermore a second hydration sphere with ∼12 water molecules at a mean Sc⋯OII distance of 4.27(3) Å. In less acidic concentrated scandium(III) aqueous solutions, the dimeric hydrolysis product, [Sc2(µ-OH)2(H2O)10]4+, is the predominating species with seven-coordinated scandium(III) ions in a double hydroxo bridge and five terminal water molecules at a mean Sc–O bond distance of 2.145 Å. Hexahydrated scandium(III) ions are found in the crystal structure of the double salt [Sc(H2O)6][Sc(CH3SO3)6], which crystallizes in the trigonal space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) with Z = 6 and the unit cell dimensions a = 14.019(2) and c = 25.3805(5) Å. The Sc–O distances in the two crystallographically unique, but nearly identical, [Sc(H2O)6]3+ entities (both with
with Z = 6 and the unit cell dimensions a = 14.019(2) and c = 25.3805(5) Å. The Sc–O distances in the two crystallographically unique, but nearly identical, [Sc(H2O)6]3+ entities (both with ![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) imposed crystallographic symmetry) are 2.085(6) and 2.086(5) Å, while the mean Sc–O distance in the near octahedral [Sc(OSO2CH3)6]3− entities (with three-fold symmetry) is 2.078 Å.
imposed crystallographic symmetry) are 2.085(6) and 2.086(5) Å, while the mean Sc–O distance in the near octahedral [Sc(OSO2CH3)6]3− entities (with three-fold symmetry) is 2.078 Å.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) with Z = 6 and the unit cell dimensions a = 14.019(2) and c = 25.3805(5) Å. The Sc–O distances in the two crystallographically unique, but nearly identical, [Sc(H2O)6]3+ entities (both with
with Z = 6 and the unit cell dimensions a = 14.019(2) and c = 25.3805(5) Å. The Sc–O distances in the two crystallographically unique, but nearly identical, [Sc(H2O)6]3+ entities (both with ![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) imposed crystallographic symmetry) are 2.085(6) and 2.086(5) Å, while the mean Sc–O distance in the near octahedral [Sc(OSO2CH3)6]3− entities (with three-fold symmetry) is 2.078 Å.
imposed crystallographic symmetry) are 2.085(6) and 2.086(5) Å, while the mean Sc–O distance in the near octahedral [Sc(OSO2CH3)6]3− entities (with three-fold symmetry) is 2.078 Å.

 Please wait while we load your content...
Please wait while we load your content...