Investigations of the hygroscopic properties of ammonium sulfate and mixed ammonium sulfate and glutaric acid micro droplets by means of optical levitation and Raman spectroscopy
Abstract
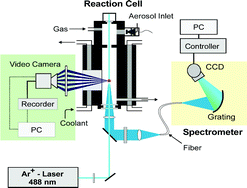
In the presented work an optical levitation technique performed by means of a focused laser beam, Mie and

 Please wait while we load your content...
Please wait while we load your content...