Determination of the temperature and pressure dependence of the reaction OH + C2H4 from 200–400 K using experimental and master equation analyses
Abstract
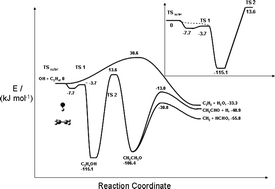
The pressure and temperature dependence for the reaction of OH + C2H4 was studied over the range of conditions: 200–400 K and 5–600 Torr by

 Please wait while we load your content...
Please wait while we load your content...