Tetraethyl stannane: structure, conformations, and orientational order when dissolved in a nematic liquid crystalline solvent
Abstract
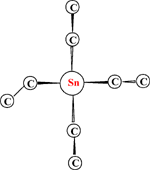
Quantum chemical calculations are used to investigate the structure and the distribution of conformers of tetraethyl stannane, and these are compared with those of

 Please wait while we load your content...
Please wait while we load your content...