On the coordination behaviour of NO3- in coordination compounds with Ag+
Part 1. Solubility effect on the formation of coordination polymer networks between AgNO3 and L
(L
= ethanediyl bis(isonicotinate) as a function of solvent
Abstract
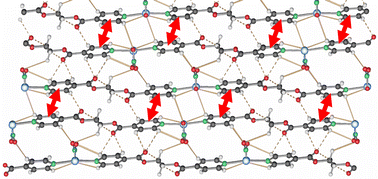
The influence of the solubility of AgNO3 in three solvent systems is studied for the reaction between AgNO3 and the

 Please wait while we load your content...
Please wait while we load your content...