Synthesis and crystal engineering of fluorinated stilbenes
Abstract
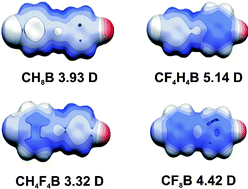
Three new polyfluorinated (E)-4-(4-bromostyryl)benzonitriles were obtained by the Horner–Wadsworth–Emmons approach to investigate intermolecular interactions in the crystalline state and the formation of co-crystals. Electrostatic surface potentials and dipole moments were calculated by ab initio methods. Three types of main intermolecular interactions were addressed to be used as a driving force to form polar co-crystals: CN⋯Br interactions lead to chain formation. Stacking of F-aromatic and H-aromatic rings is present. C–F⋯H and C–F⋯F interactions were identified, which are weak driving forces to align molecules sidewise. Crystal structures of the three stilbenes are centric. By formation of solid solutions [(E)-4-(4-bromostyryl)-2,3,5,6-tetrafluorobenzonitrile]x·[(E)-4-(4-bromo-2,3,5,6-tetrafluorostyryl)benzonitrile]1−x, polar properties were observed. In the case of (E)-4-(4-bromostyryl)benzonitrile·(E)-4-(4-bromo-2,3,5,6-tetrafluorostyryl)-2,3,5,6-tetrafluorobenzonitrile, a non-polar 1 ∶ 1 ordered structure is formed.

 Please wait while we load your content...
Please wait while we load your content...