Supramolecular aggregation in diimine adducts of zinc(ii) dithiophosphates: controlling the formation of monomeric, dimeric, polymeric (zig-zag and helical), and 2-D motifs
Abstract
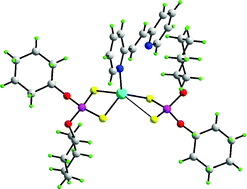
The interplay between steric demands of dithiophosphate-bound R groups in Zn(S2P(OR)2)2 on the one hand and the coordination requirements of a variety of di-pyridyl-type bases on the other, is shown to be pivotal in determining supramolecular aggregation patterns in a series of their adducts. Thus, the combination of sterically demanding cyclohexyl groups and the congested ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)C5H4N-2)0.5]2; a similar motif is found when
C(H)C5H4N-2)0.5]2; a similar motif is found when

 Please wait while we load your content...
Please wait while we load your content...