Synthesis of propargylic fluorides from allenylsilanes†
Abstract
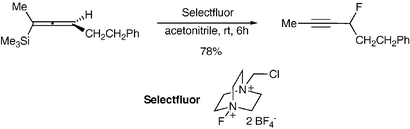
Allenylsilanes reacted at room temperature in acetonitrile with Selectfluor, an electrophilic fluorinating reagent, to give secondary propargylic fluorides in moderate to good yields; mechanistically, a side-product resulting from a 1,2-silyl shift testifies to the presence of a cationic intermediate.

 Please wait while we load your content...
Please wait while we load your content...