Annulation of β-aryl-α-nitro-α,β-enals and 2,2-dimethyl-1,3-dioxan-5-one: a one-step assembly of nitrocyclitols. Application to a short practical synthesis of (±)-7-deoxy-2-epi-pancratistatin tetraacetate†
Abstract
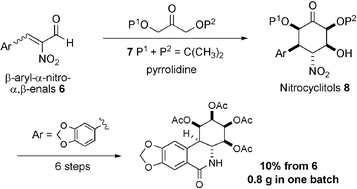
A novel, highly stereocontrolled formal [3 + 3] annulation of β-aryl-α-nitro-α,β-enals with the

 Please wait while we load your content...
Please wait while we load your content...