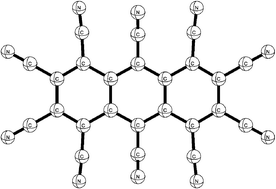
The optimised structures, electron affinities, and vibrational frequencies of the simplest benzenoid cyanocarbons, namely hexacyanobenzene C6(CN)6, octacyanonaphthalene C10(CN)8, and decacyanoanthracene C14(CN)10, have been studied using carefully calibrated density functional methods (Chem. Rev., 2002, 102, 231–282); the predicted adiabatic electron affinities are 3.53 eV for C6(CN)6, 4.35 eV for C10(CN)8 and 5.02 eV for C14(CN)10, which are significantly larger than those of the analogous benzenoid fluorocarbons as well as tetracyanoethane and tetracyanoquinodimethane.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...