The Morita–Baylis–Hillman adducts of β-aryl nitroethylenes with other activated alkenes: synthesis and anticancer activity studies†‡
Abstract
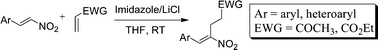
The Morita–Baylis–Hillman (MBH) adducts of β-aryl nitroethylenes with
- This article is part of the themed collection: 20th Anniversary of the CRSI: Celebrating Indian Chemistry in ChemComm

 Please wait while we load your content...
Please wait while we load your content...