Site-specific amide hydrogen exchange in melittin probed by electron capture dissociation Fourier transform ion cyclotron resonance mass spectrometry
Abstract
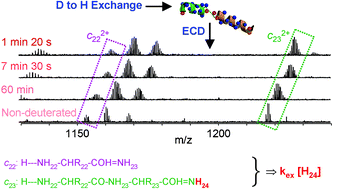
Electron capture dissociation (ECD) has been proposed to be a non-ergodic process, i.e. to provide backbone dissociation of gas-phase peptides faster than randomization of the imparted energy. One potential consequence could be that ECD can fragment deuterated peptides without causing hydrogen scrambling and thereby provide amino acid residue-specific amide hydrogen exchange rates. Such a feature would improve the resolution of approaches involving solution-phase amide hydrogen exchange combined with mass spectrometry for protein structural characterization. Here, we explore this hypothesis using melittin, a haemolytic polypeptide from bee venom, as our model system. Exchange rates in methanol calculated from consecutive c-type ion pairs show some correlation with previous NMR data: the amide hydrogens of leucine 13 and alanine 15, located at the unstructured kink surrounding proline 14 in the melittin structure adopted in methanol, appear as fast exchangers and the amide hydrogens of leucine 16 and lysine 23, buried within the helical regions of melittin, appear as slow exchangers. However, calculations based on c-type ions for other amide hydrogens do not correlate well with NMR data, and evidence for deuterium scrambling in ECD was obtained from z˙-type ions.

 Please wait while we load your content...
Please wait while we load your content...