Formation of hybrid micelles between poly(ethylene glycol)-block-poly(4-vinylpyridinium) cations and sulfate anions in an aqueous milieu†
Abstract
The SO42−-induced micellization of poly(ethylene glycol)-block-poly(4-vinylpyridinium)
(PEG-b-P(4-VPH+)) in aqueous solution is studied by dynamic and static light scattering, and
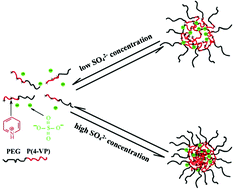

 Please wait while we load your content...
Please wait while we load your content...