Exceptionally slow kinetics of the intramolecular quadruplex formed by the Oxytricha telomeric repeat
Abstract
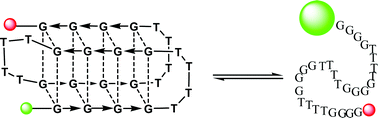
We examined the stability and kinetics of folding of the Oxytricha telomeric repeat sequence (G4T4)4. Fluorescence melting experiments show that this intramolecular quadruplex, which is more stable in potassium- than sodium-containing

 Please wait while we load your content...
Please wait while we load your content...