Studies towards the synthesis of epothilone A via organoboranes†
Abstract
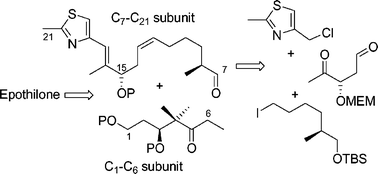
Studies towards the synthesis of epothilone A via organoboranes have been described. A modified procedure for the large-scale preparation of B-γ,γ-dimethylallyldiisopinocampheylborane from

 Please wait while we load your content...
Please wait while we load your content...