Nitric oxide release from the unimolecular decomposition of the superoxide radical anion adduct of cyclic nitrones in aqueous medium
Abstract
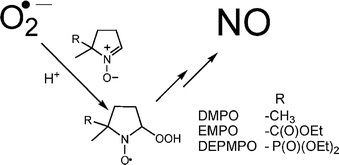
Nitrones such as 5,5-dimethyl-1-pyrroline N-oxide (DMPO), 5-diethoxyphosphoryl-5-methyl-1-pyrroline N-oxide (DEPMPO) and 5-ethoxycarbonyl-5-methyl-1-pyrroline N-oxide (EMPO) have become the spin-traps of choice for the detection of transient radical species in chemical and biological systems using electron paramagnetic resonance (EPR) spectroscopy. The mechanism of decomposition of the superoxide radical anion (O2˙−) adducts of DMPO, DEPMPO and EMPO in aqueous solutions was investigated. Our findings suggest that nitric oxide (NO) was formed during the decomposition of the O2˙− adduct as detected by EPR spin trapping using Fe(II) N-methyl-D-glucamine dithiocarbamate (MGD). Nitric oxide release was observed from the O2˙− adduct formed from hypoxanthine–xanthine oxidase, PMA-activated human neutrophils, and DMSO solution of KO2. Nitric oxide formation was not observed from the independently generated hydroxyl radical adduct. Formation of nitric oxide was also indirectly detected as nitrite (NO2−) utilizing the Griess assay. Nitrite concentration increases with increasing O2˙− concentration at constant DMPO concentration, while NO2− formation is suppressed at anaerobic conditions. Moreover, large excess of DMPO also inhibits NO2− formation which can be attributed to the oxidation of DMPO to hydroxamic acid nitroxide (DMPO–X) by nitrogen dioxide (NO2), a precursor to NO2−. Product analysis was also conducted to further elucidate the mechanism of adduct decay using gas chromatography-mass spectrometry (GC-MS) technique.

 Please wait while we load your content...
Please wait while we load your content...