Synthesis of (6R)- and (6S)-5,10-dideazatetrahydrofolate oligo-γ-glutamates: Kinetics of multiple glutamate ligations catalyzed by folylpoly-γ-glutamate synthetase†
Abstract
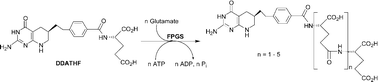
Folylpoly-γ-glutamate synthetase (FPGS, EC 6.3.2.17) catalyzes the ATP-dependent ligation of glutamic acid to reduced folates including (6S)-5,6,7,8-tetrahydrofolate (H4PteGlu), as well as to anticancer drugs such as 5,10-dideaza-5,6,7,8-tetrahydrofolate ((6R)-DDAH4PteGlu1, (6R)-DDATHF, Lometrexol™). Synthesis of unlabeled mono- and polyglutamates, DDAH4PteGlun (6R, n = 1–6; 6S, n = 1–2), as well as (6R)-DDAH4Pte[14C]Glu1, was effected from (6R)- or (6S)-5,10-dideazatetrahydropteroyl azide and glutamic acid, H-Glu-γ-Glun-γ-Glu-OH (n = 0–4), or [14C]glutamic acid, respectively. These compounds were evaluated as FPGS substrates to determine steady-state kinetic constants. Michaelis–Menten kinetics were observed for (6R)-DDAH4PteGlu1, the isomer corresponding to H4PteGlu, whereas marked substrate inhibition was observed for (6S)-DDAH4PteGlun (n = 1–2) and (6R)-DDAH4PteGlun (n = 2–5), but not (6R)-DDAH4PteGlu6. Multiple ligation of glutamate renders a quantitative analysis of these data difficult. However, approximate values of KM = 0.65–1.6 µM and KI = 144–417 µM for DDAH4PteGlun were obtained using a simple kinetic model.

 Please wait while we load your content...
Please wait while we load your content...