Synthesis and biological evaluation of novel fumagillin and ovalicin analogues†
Abstract
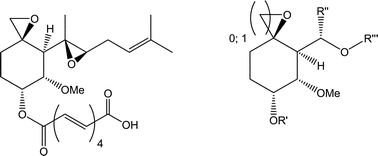
A promising approach among the numerous efforts to cure cancer is the interruption of the tumour-induced formation of new blood vessels (angiogenesis). By suppressing angiogenesis with

 Please wait while we load your content...
Please wait while we load your content...