Cyclohexane bis-urea compounds for the gelation of water and aqueous solutions†
Abstract
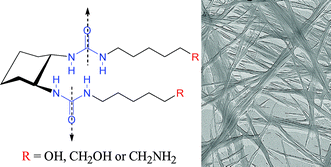
A new class of efficient hydrogelators has been developed by a simple modification of the peripheral substituents of cyclohexane bis-urea organogelators with hydrophilic

 Please wait while we load your content...
Please wait while we load your content...