Conformational properties of peptide fragments homologous to the 106–114 and 106–126 residues of the human prion protein: a CD and NMR spectroscopic study †‡
Abstract
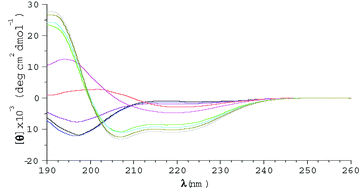
Two peptide fragments, corresponding to the amino acid residues 106–126 (PrP[Ac-106–126-NH
2]) and 106–114 (PrP[Ac-106–114-NH
2]) of the human prion

 Please wait while we load your content...
Please wait while we load your content...