The formation and properties of the melatonin radical: a photolysis study of melatonin with 248 nm laser light
Abstract
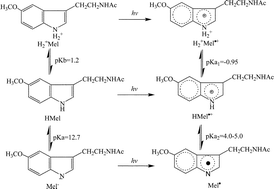
The photolysis of melatonin in aqueous solution has been studied spectrometrically with a 248 nm laser. The formation of hydrated electrons in a monophotonic process has been confirmed in neutral solution with a quantum yield of 0.22. Two main absorption bands at 340 and 460 nm plus an absorption shoulder resulted from the counterpart of the ejected electron, a melatonin radical, in solution. The big difference for the relative intensity of the absorption peaks under various pH conditions reveals that the melatonin radical exists in the solution through an acid–base equilibrium. In support from the pH dependence of the spectrum of the intermediate, the pKa1 for the doubly-protonated melatonin radical against the mono-protonated melatonin cation radical was estimated to be −0.95 and the pKa2 for the mono-protonated melatonin cation and melatonin neutral radical was 4.5 ± 0.5. This work will benefit the basic understanding about melatonin as a UV-light protector, as a light receptor and the antioxidation functions of melatonin.

 Please wait while we load your content...
Please wait while we load your content...