Simple, versatile and highly diastereoselective synthesis of 1,3,4-trisubstituted-2-oxopiperazine-containing peptidomimetic precursors
Abstract
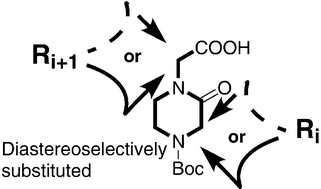
The selective O-deprotection of (1′S)-4-(tert-butoxycarbonyl)-1-[1′-phenylmethyloxymethyl-2′-[(tert-butyldimethylsilyl)oxy]ethyl]-2-oxopiperazine furnished an enantiomerically pure alcohol whose regio- and diastereoselective C3-alkylation yielded either (3R)- or (3S)-1,3,4-trisubstituted-2-oxopiperazines in high diastereomeric purity. These derivatives were efficiently transformed into (1′R)- or (1′S)-peptide templates utilizable to prepare peptidomimetics. This method provides easy access to each 1,3,4-trisubstituted-2-oxopiperazine diastereomer and facilitates, through the large choice of substituents at the 3-position together with the chemistry that can be performed on the N1 substituent, the preparation of a large number of diastereomerically pure constrained peptidomimetics from a single precursor.

 Please wait while we load your content...
Please wait while we load your content...