Modelling protein–RNA interactions: an electron density study of the guanidinium and formate complexes with RNA bases†
Abstract
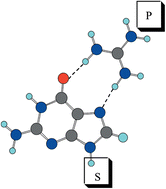
The complexes formed by the double interaction established between RNA bases and guanidinium and formate ions, as a model for the interacting groups of arginine and glutamic or aspartic amino acid side chains, have been theoretically studied. A density functional theory method (B3LYP/6-31 + G**) has been used for this study. The range of interaction energies obtained allowed for a distinction between bidentate and bifurcate hydrogen bond interactions. The analysis of the electron density and the natural bond orbital analysis shows that these complexes are bound by double hydrogen bonds established between the donor and acceptor groups of guanidinium and formate respectively and those of the RNA bases. Comparisons are made with the results obtained in some previous theoretical and experimental studies.

 Please wait while we load your content...
Please wait while we load your content...