Total synthesis of the Fusarium toxin equisetin
Abstract
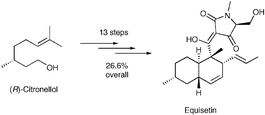
A short stereoselective synthesis of the Fusarium toxin equisetin, a potent inhibitor of HIV-1 integrase enzyme is described, using as the key step a stereoselective intramolecular Diels–Alder reaction of a fully conjugated E,E,E-triene with a trisubstituted γ,δ-unsaturated β-ketothioester.

 Please wait while we load your content...
Please wait while we load your content...