Energetic, environmental and economic balances: Spice up your ionic liquid research efficiency†
Abstract
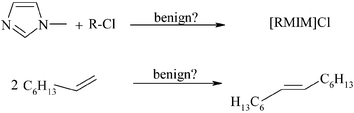
The energy requirement, environmental impact and material costs of the synthesis of ionic liquids, and of their subsequent use as reaction media in the metathesis of 1-octene, are compared to conventional solvents. This preliminary study lays the foundation for an ecological and strategic experimental design. Energetic, environmental and economic assessments over all life-cycle stages allow for the identification of both, disadvantages and opportunities of individual process steps, at an early R&D level. Thus, this approach helps to find new and improved solutions, which comply with the concepts of “Green Chemistry”, that cannot be determined by experimental work alone. The potential of innovative methods can be quantitatively compared to current technologies by means of the energy efficiency factor EEF. Interestingly, this study demonstrates that under certain circumstances, a solvent-free reaction mode may not necessarily be ecologically advantageous. Also, the presumption that, due to facile recycling, a bi-phasic reaction mode is always superior to a homogeneous one is questioned: compared to the energy required for the manufacture of a solvent which results in a biphasic reaction mode (e.g. an ionic liquid), the energy needed for the separation of a homogeneous reaction mixture by distillation is comparatively small. Thus, efficient recycling of such a solvent must be guaranteed.

 Please wait while we load your content...
Please wait while we load your content...