Structural and zeolitic features of a series of heterometallic supramolecular porous architectures based on tetrahedral {M(C2O4)4}4− primary building units†
Abstract
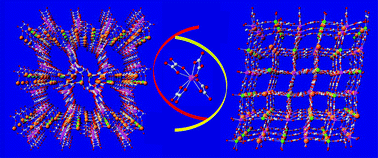
The utilization of tetrahedral pre-formed coordination compounds {M(C2O4)4}4−
(M = ZrIV, UIV; C2O42−
= oxalate) permitted the efficient construction of rare examples of heteronuclear supramolecular nano-porous architectures. A series of metal–organic coordination frameworks prepared by association of these building units with either Mn2+, Cd2+, or Mg2+ have been structurally characterized and are described. Their 3-D chemical scaffold is based on the primary tetrahedral building unit but their pore sizes and topologies could be varied through the M2+ metal ion involved in the assembling process, and the anionic tetrahedral moiety. These structures display channels with apertures up to 12 Å
× 8 Å which are emptied of solvates at mild temperatures without affecting the chemical scaffold, the integrity of which is maintained up to 250–300 °C. A certain degree of flexibility of the coordination

 Please wait while we load your content...
Please wait while we load your content...