Platinum(ii) 1,10-phenanthroline complexes of acetylides containing redox-active groups†
Abstract
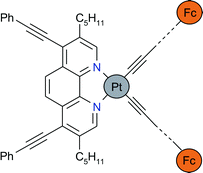
The new diimine ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–Ph)2], [Pt(1)(C
C–Ph)2], [Pt(1)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–Fc)2] and [Pt(1)(C
C–Fc)2] and [Pt(1)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–p-C6H4–C
C–p-C6H4–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–Fc)2]
(Fc = ferrocenyl). Crystal structure analyses were performed for [Pt(1)Cl2] and [Pt(1)(C
C–Fc)2]
(Fc = ferrocenyl). Crystal structure analyses were performed for [Pt(1)Cl2] and [Pt(1)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–Ph)2] and revealed that the di(acetylide)
π-tweezer of the latter binds a molecule of chloroform through C–H⋯π hydrogen bonds. The redox and optical properties of 1 and its complexes were investigated by (spectro-)electrochemistry, UV–Vis and luminescence spectroscopy, and an energy level diagram was derived for [Pt(1)(C
C–Ph)2] and revealed that the di(acetylide)
π-tweezer of the latter binds a molecule of chloroform through C–H⋯π hydrogen bonds. The redox and optical properties of 1 and its complexes were investigated by (spectro-)electrochemistry, UV–Vis and luminescence spectroscopy, and an energy level diagram was derived for [Pt(1)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–Fc)2] and related compounds on the basis of the data collected. The ferrocenyl-substituted PtII complexes are donor–sensitiser assemblies. Intramolecular quenching of the photoexcited PtII
C–Fc)2] and related compounds on the basis of the data collected. The ferrocenyl-substituted PtII complexes are donor–sensitiser assemblies. Intramolecular quenching of the photoexcited PtII ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–p-C6H4–C
C–p-C6H4–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–Fc)2]
(2 ns) and [Pt(1)(C
C–Fc)2]
(2 ns) and [Pt(1)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–Fc)2]
(0.3 ns), as opposed to [Pt(1)(C
C–Fc)2]
(0.3 ns), as opposed to [Pt(1)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–Ph)2]
(0.7 µs). Excimer formation has been observed for [Pt(1)(C
C–Ph)2]
(0.7 µs). Excimer formation has been observed for [Pt(1)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–Ph)2] at room temperature in
C–Ph)2] at room temperature in

 Please wait while we load your content...
Please wait while we load your content...