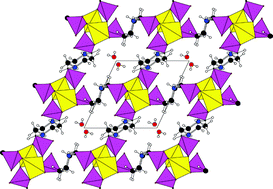
An exploration of the reactions of N,N′-piperazinebis(methylenephosphonic acid), H4L, with zinc salts has led to the isolation of two new framework zinc phosphonates. ZnLH2·H2O (I) is isostructural with previously reported manganese(II) and cobalt(II) analogues, and consists of infinite ‘zinc phosphate’ chains bridged into three dimensions via the organic moieties. The resulting framework encloses large channels in which the loosely bound H2O resides. The H2O is lost reversibly at around 160 °C, without framework collapse. Zn2L (II) has a novel framework structure, prepared at an initial pH > 7, which consists of two-dimensional ‘zinc phosphate’ sheets, comprising both four- and eight-membered –Zn–O–P– rings, which are also linked into three dimensions via the organic groups. In both cases, the zinc centre is tetrahedral; in I coordination is by oxygen atoms from four different phosphonate groups, whereas in II the additional deprotonation of the ligand allows coordination via three oxygen atoms plus the amine nitrogen atom.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...