A medium-nuclearity mixed-valence polyoxomolybdate [H2Mo16O52]10−
=
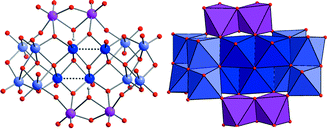
{Mo16}
(1a) was synthesized using an approach that employed protonated hexamethylenetetramine (HMTAH+) as counter ion and yielded (HMTAH)101a·34 H2O (1). The {Mo16} cluster anion exhibits significant nucleophilicity and traps electrophiles such as divalent transition metal ions, resulting in a family of isostructural compounds based on {Mo16M2}-type anions [M(H2O)8H2Mo16O52]6−
(M = FeII
(2), MnII
(3), CoII
(4)). The highly reactive nature of the {Mo16} system is also revealed by rearrangement and decomposition reactions of 1 to either slowly form a sodium-bridged heptamolybdate-based chain compound (5) when left in the reaction solution or, in the presence of very high concentrations of electrophiles, to heptamolybdate-based cluster compounds [M2(H2O)9Mo7O24]2− of the {M2Mo7}-type (M = FeII
(6), MnII
(7)). Compounds 1–7 were characterised by single crystal X-ray diffraction, elemental analysis, IR spectroscopy, magnetic susceptibility measurements, and density functional theory calculations.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...