Exchange coupling in μ-aqua:μ-oxo vs. di-μ-hydroxo dinuclear Cu(ii) compounds: a density functional study
Abstract
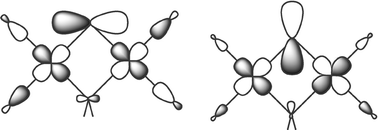
Theoretical methods based on density functional theory have been applied to study the differences in exchange coupling between the di-μ-hydroxo and μ-aqua:μ-oxo tautomers of a dinuclear Cu(II) complex. The calculations indicate that the two compounds are totally different from a magnetic point of view. The transfer of one

 Please wait while we load your content...
Please wait while we load your content...