Role of sulfur chirality in the chemical processes of biology
Abstract
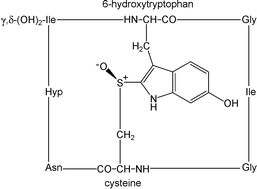
Chiral structures profoundly influence chemical and biological processes. While chiral carbon biomolecules have received much attention, chirality is also possible in certain sulfur compounds; just as with carbon, there can be differences in the physiological behavior of chiral sulfur compounds. For instance, one drug enantiomer, Nexium® (esomeprazole, a chiral sulfoxide), is used for its superior clinical properties as a proton pump inhibitor over the racemic mixture, Prilosec® (Losec®, omeprazole). This critical review introduces sulfur stereochemistry and nomenclature, and provides a comprehensive approach to chiral sulfur compounds and their enzymatic reactions in general and secondary metabolism. The major structural types of biological interest are sulfonium salts, sulfoxides, and sulfoximines. (103 references)

 Please wait while we load your content...
Please wait while we load your content...