Salts of extended tetrathiafulvalene analogues: relationships between molecular structure, electrochemical properties and solid state organisation
Abstract
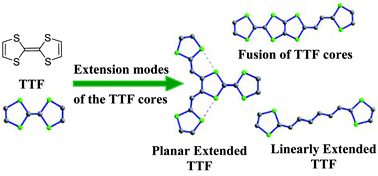
By considering the structures of many salts derived from extended TTF analogues, relationships between the molecular architecture of the donors with their electrochemical properties and their stacking mode in the salts are presented in this critical review. Three categories of donors corresponding to their extension modes have been considered. Firstly, for linearly extended TTFs the crucial role of the spacer in modifying the electrochemical properties and the packing mode in the salts is presented. Secondly, bidimentional extension of the donors obtained by linking several dithiafulvenyl units on a TTF core led to materials with increased dimensionality. Finally, the last class corresponds to the fusion, directly or across a

 Please wait while we load your content...
Please wait while we load your content...