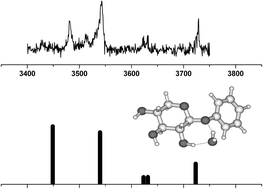
The gas phase structures of phenyl α- and β-D-xylopyranoside (α- and β-pXyl) and their mono-hydrates have been investigated using a combination of resonant two-photon ionization (R2PI), ultra-violet hole-burning and resonant infrared ion dip spectroscopy, coupled with density functional theory (DFT) and ab initio computation. The hole-burning experiments indicate the population of a single conformer only, in each of the two anomers. Their experimental and calculated infrared spectra are both consistent with a conformational assignment corresponding to the computed global minimum configuration. All three OH groups are oriented towards the oxygen atom (O1) on the anomeric carbon atom to form an all trans
(ttt) counter-clockwise chain of hydrogen bonds. The mono-hydrates, α- and β-pXyl(H2O) each populate two distinct structures in the molecular beam environment, with the water molecule inserted between OH4 and OH3 or between OH3 and OH2 in α-pXyl(H2O), and between OH2 and O1 in either of two alternative orientations, in β-pXyl(H2O). In all of the mono-hydrated xyloside complexes, the water molecule inserts into the weakest link of the sugar molecules’ hydrogen-bonded chain of hydroxy groups, creating a single extended chain, strengthened by co-operativity. The all-trans configuration of the xylose moiety is retained and the mono-hydrate structures correspond to those calculated to lie at the lowest relative energies.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...