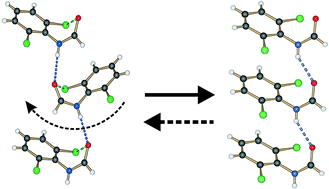
The crystal structures, thermal behaviour and phase transformations of a series of 2,6-disubstituted-N-phenylformamides have been investigated. A phase transformation was only observed when chlorine was one of the substituents. Crystals of the room-temperature form of 2,6-dichloro-N-phenylformamide (1) and 2-chloro-6-methyl-N-phenylformamide (2) are isomorphous. Both compounds are orthorhombic at room temperature and transform to a monoclinic high-temperature form at 155 and 106 °C, respectively. The room-temperature structures of 1 and 2 consist of chains of N–H⋯O hydrogen-bonded molecules stacked in an alternating arrangement along the crystallographic a direction. The high-temperature forms of compounds 1 and 2 (grown by sublimation) are both monoclinic but not isomorphous, with one short axis of about 4.3 Å, and consist of chains of N–H⋯O hydrogen-bonded molecules stacked along the short axis, related by translation. When both of the chlorine substituents are replaced by methyl groups, as in 2,6-dimethyl-N-phenylformamide (3), the crystals do not undergo any phase transition on heating and only an orthorhombic form, space group P212121, has been isolated. Examination of the molecular geometry and structural properties of 3 indicates that it is a hybrid structure of the low- and high-temperature forms of compounds 1 and 2. This contribution analyzes the effect of chloro-methyl exchange, the steering ability of chlorine, and the role of weak interactions on the structural and thermal properties of the compounds studied. In addition, a mechanism for the phase change in 1 is proposed and rationalized through the examination of the structures themselves together with lattice energy calculations.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...