Structure–function correlations in oxygen activating non-heme iron enzymes
Abstract
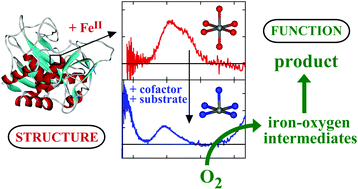
A large group of mononuclear non-heme iron enzymes exist which activate dioxygen to catalyze key biochemical transformations, including many of medical, pharmaceutical and environmental significance. These enzymes utilize high-spin FeII active sites and additional reducing equivalents from cofactors or substrates to react with O2 to yield iron-oxygen intermediates competent to transform substrate to product. While FeII sites have been difficult to study due to the lack of dominant spectroscopic features, a spectroscopic methodology has been developed which allows the elucidation of the geometric and electronic structures of these active sites and provides molecular level insight into the mechanisms of catalysis. This review provides a summary of this methodology with emphasis on its application to the determination of important active site structure–function correlations in mononuclear non-heme iron enzymes. These studies provide key insight into the mechanisms of oxygen activation, active site features that contribute to differences in reactivity and, combined with theoretical calculations and model studies, the nature of oxygen intermediates active in catalysis.

 Please wait while we load your content...
Please wait while we load your content...