Exploiting C3-symmetry in the dynamic coordination of a chiral trisoxazoline to copper(ii): improved enantioselectivity, and catalyst stability in asymmetric lewis acid catalysis†
Abstract
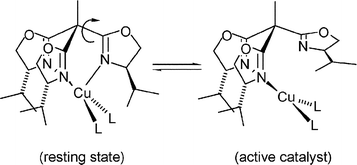
Chiral C3-symmetric trisoxazolines are highly efficient stereodirecting

 Please wait while we load your content...
Please wait while we load your content...