Towards the combinatorial synthesis of spongistatin fragment libraries by using asymmetric aldol reactions on solid support†
Abstract
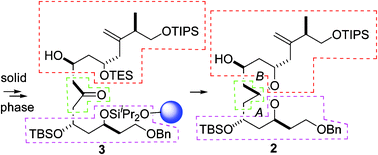
By relying on asymmetric boron-mediated aldol reactions, solid phase methodology for the stereoselective synthesis of highly substituted spiroacetals was developed and applied to the preparation of a complex AB-spiroacetal subunit of the

 Please wait while we load your content...
Please wait while we load your content...