Unprecedented, fully recyclable, solid-supported reagent for the kinetic resolution of racemic amines through enantioselective N-acetylation†
Abstract
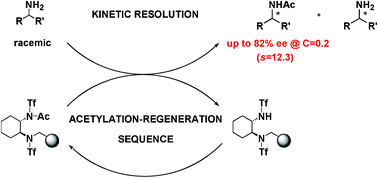
The high-yielding synthesis and application of the first polymer supported reagent for the kinetic resolution (KR) of amines through enantioselective acetylation is described; this new supported chiral reagent allows the KR of primary amines with excellent selectivities at room temperature; moreover, this supported approach is highly efficient as the Merrifield-supported chiral scaffold can be quantitatively recovered and recycled.

 Please wait while we load your content...
Please wait while we load your content...