A novel strategy for the asymmetric synthesis of chiral cyclopropane carboxaldehydes†
Abstract
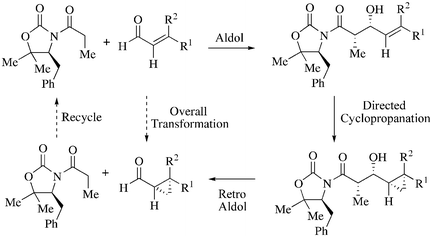
A new way of combining chiral auxiliaries and substrate-directable reactions for asymmetric synthesis is described that employs a three-step sequence of aldol–cyclopropanation–retro-aldol reactions for the stereoselective synthesis of enantiopure cyclopropane carboxaldehydes.

 Please wait while we load your content...
Please wait while we load your content...