Identification of new N–Sb topologies: understanding the sequential dehydrochloride coupling of primary amines and trichloropnictines
Abstract
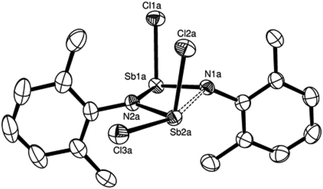
Subtle steric strain imposed by 2,6-dimethylphenyl substituents on N–Sb frameworks has enabled identification of the first acyclic dipnictadiazane and the first six-membered cyclotristibatriazane providing insight into the dehydrohalide coupling reaction of

 Please wait while we load your content...
Please wait while we load your content...