Imprinted polymers for chiral resolution of (±)-ephedrine: understanding the pre-polymerisation equilibrium and the action of different mobile phase modifiers
Abstract
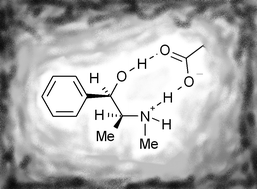
A thorough study has been made of the ephedrine–methacrylic acid (MAA) system for molecular imprinting, involving NMR studies of the pre-polymerisation equilibria, modelling and HPLC enantioseparations with different mobile phases. When dimerisation of MAA is accounted for, NMR titrations demonstrate there is a very strong (‘stoichiometric’, K ≈ 10000 M−1) interaction between ephedrine and a single MAA monomer. Polymers prepared with a 1 ∶ 1 monomer ∶ template ratio are capable of enantioseparation, indicating, in combination with the NMR results, that the 1 ∶ 1 interaction probably involves the carboxylic acid acting as a chelating monomer, forming hydrogen bonds to both the template amine and hydroxyl moieties. Higher monomer ∶ template ratios cause further changes in the NMR signals, suggesting at least one further MAA can interact with the amine group, with a weaker association constant (K ≈ 80 M−1). Polymers prepared with a 4 ∶ 1 monomer ∶ template ratio are thus proposed to contain a mixture of 1-monomer binding sites and 2-monomer binding sites, the latter being of enhanced acidity. In HPLC, better results are obtained with the 4 ∶ 1 polymer using acetic acid as a modifier, while better results are obtained for the 1 ∶ 1 polymer using butylamine as a modifier. We propose a model whereby acetic acid exerts its effect by reducing binding to the 1-monomer sites, while butylamine works largely through blocking the most acidic, 2-monomer sites. For preparative chromatography we suggest the 1 ∶ 1 polymer, with a larger population of weaker but more uniform binding sites, is most promising.

 Please wait while we load your content...
Please wait while we load your content...