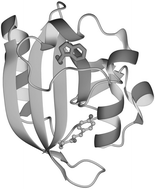
Photoactive yellow protein, a small, water-soluble blue-light absorbing photoreceptor protein from Ectothiorhodospira
(Halorhodospira) halophila has a structure with two hydrophobic cores, of which the main one houses its light-sensitive chromophore (p-coumaric acid), separated by a central β-sheet. This photoreceptor protein contains a single tryptophan residue (W119) that is situated at the interface between the central β-sheet and its N-terminal cap. The fluorescence properties of W119 in the dark state pG (λmax
= 328 nm; Φfl
= 0.01; nearly pH-independent) are typical for a buried tryptophan in a hydrophobic environment with significant quenching by nearby amino acid residues. Signalling state formation leads to pH-dependent fluorescence changes: At pH values <6.5 the fluorescence emission increases, with a minor blue shift of the emission maximum. Above this pH, the emission maximum of the tryptophan shifts considerably to the red, whereas its total intensity decreases. These results further support the contention that signalling state formation in PYP leads to significant changes in the structure of this protein, even at sites that are at a considerable distance from the chromophore. The nature of these changes in pB, however, depend upon the pH imposed upon the protein: At slightly alkaline pH, which presumably is closest to the pH to which this protein is exposed in vivo, these changes lead to an exposure of the part of the central β-sheet harbouring W119. At slightly acidic pH the polarity of the environment of W119 is hardly affected by the formation of the signalling state but the quenching of its fluorescence emission, possibly by nearby amino acids, is reduced. On the other hand, its accessibility for quenching by small molecules in the solution is enhanced at acidic and alkaline pH in the signalling state (pB) compared to the dark state (pG). This latter observation points towards a more flexible structure of the N-terminal cap, having a looser interaction with the central β-sheet in pB.

 Please wait while we load your content...
Please wait while we load your content...