Synthesis of the tetraethyl substituted pH-sensitive nitroxides of imidazole series with enhanced stability towards reduction
Abstract
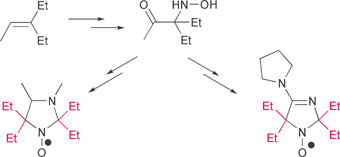
The synthesis of 2,2,5,5-tetraethylimidazole nitroxides from 3-ethylpent-2-ene is described. The newly synthesized nitroxides, namely 4-methyl-2,2,5,5-tetraethyl-2,5-dihydro-1H-imidazol-1-yloxy (1), 3,4-dimethyl-2,2,5,5-tetraethylperhydroimidazol-1-yloxy (2) and 2,2,5,5-tetraethyl-4-pyrrolidin-1-yl-2,5-dihydro-1H-imidazol-1-oxyl (3), were found to be pH sensitive spin probes, with pK values of 1.2, 4.95 and 7.4, respectively. The most important finding was the fact that these new nitroxides were 20–30 times more stable in the presence of ascorbate and had significantly longer halflifes in rat blood as compared to 2,2,5,5-tetramethyl analogs. The latter observation provides a unique advantage for the application of tetraethyl substituted imidazole nitroxides as functional EPR probes.

 Please wait while we load your content...
Please wait while we load your content...