Nucleophilic trifluoromethylation of cyclic imides using (trifluoromethyl)trimethylsilane CF3SiMe3†
Abstract
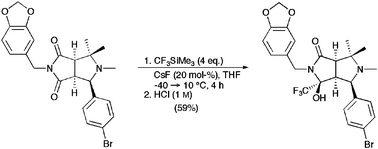
A variety of cyclic five-membered imides was trifluoromethylated in good to excellent chemical yields using (trifluoromethyl)trimethylsilane CF3SiMe3 under fluoride ion catalysis. The method was successfully applied to the stereoselective synthesis of trifluoromethylated bi- and tricyclic lactams which could serve as precursors for designed thrombin inhibitors.

 Please wait while we load your content...
Please wait while we load your content...