Phorboxazole B synthetic studies: construction of C(1–32) and C(33–46) subtargets†
Abstract
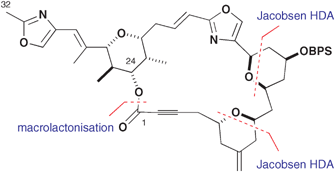
The convergent syntheses of the C(1–32) and C(33–46) domains of phorboxazole B are described. An iterative cyclocondensation strategy exploited the Jacobsen hetero-Diels–Alder (HDA) reaction as a platform for the synthesis of both the C(5–9) and C(11–15) tetrahydropyran rings. The use of 2-silyloxydiene coupling partners bearing an increasing resemblance to the phorboxazole skeleton was found to lead to a reduction in diastereoselectivity, however, in the case of the C(11–15) ring. The coupling of

 Please wait while we load your content...
Please wait while we load your content...