Highly-functionalised difluorinated (hydroxymethyl)conduritol analogues via the Diels–Alder reactions of a difluorinated dienophile
Abstract
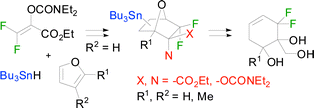
A difluorodienophile, synthesised using a Stille coupling reaction underwent tin(IV)-catalysed cycloaddition with three furans to afford oxa[2.2.1]bicycloheptenes in good yield. Reduction of ester and carbamate carbonyl groups and diol protection as the acetonide set the stage for palladium-catalysed hydrostannylation in two cases. Treatment of the stannanes with methyllithium triggered ring-opening to afford highly-functionalised difluorinated cyclohexenols which could be deprotected to afford (hydroxymethyl)conduritol analogues.

 Please wait while we load your content...
Please wait while we load your content...