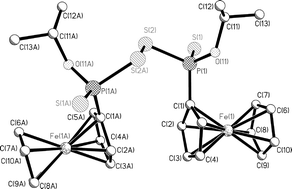
Reaction of Fc(S)PS2P(S)Fc and An(S)PS2P(S)An with NaOR [R = Me, Et, iPr] gives the non symmetric sodium phosphonodithioate salts Fc(RO)PS2Na and An(RO)PS2Na respectively (An = anisyl, Fc = ferrocenyl). These salts can be oxidised by I2, activated by KI, to form organodithiophosphono disulfides of the type (R(R′O)P(S)–S–S–P(S)(OR′)R). Reactions of these salts with 2,4-dinitrosulfenyl chloride form organodithiophosphono disulfides of the type [R(R′O)P(S)–S–S–R]. These sodium salts can also react with benzyl bromide to form S-alkyl O-alkyl 4-methoxyphenyldithiophosphonate esters and S-alkyl O-alkyl ferrocenyldithiophosphonate esters. All new compounds have been characterised spectroscopically and seven demonstrative X-ray structures are reported.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...