Oxidation of non-phenolic substrates with the laccase/N-hydroxyacetanilide system: Structure of the key intermediate from the mediator and mechanistic insight
Abstract
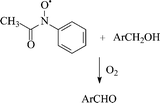
We have investigated the reactivity and mechanistic features in the oxidation of non-phenolic substrates by the enzyme laccase under mediation by ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–O˙ reactive intermediate of the mediator, formed in a preliminary oxidative interaction with the enzyme, seems the more viable
N–O˙ reactive intermediate of the mediator, formed in a preliminary oxidative interaction with the enzyme, seems the more viable ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–O˙ intermediate of NHA. The occurrence of an alternative ionic route through the oxoammonium ion (
N–O˙ intermediate of NHA. The occurrence of an alternative ionic route through the oxoammonium ion (![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N
N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O+) of mediator NHA is ruled out by experimental evidence acquired through an Hammett structure/reactivity correlation in the oxidation of substituted
O+) of mediator NHA is ruled out by experimental evidence acquired through an Hammett structure/reactivity correlation in the oxidation of substituted ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N
N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O+ species of NHA, being a one-electron oxidant of moderate strength, could in principle even be responsible for an alternative electron-transfer route of
O+ species of NHA, being a one-electron oxidant of moderate strength, could in principle even be responsible for an alternative electron-transfer route of

 Please wait while we load your content...
Please wait while we load your content...