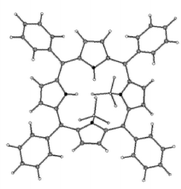
The interaction of a series of free base meso-tetraarylporphyrins (arylpor) and meso-tetraalkylporphyrins (alkylpor) with BF3·Et2O, with different molar ratios (<1∶1 to >1∶2) in chloroform, immediately and exclusively yielded the 1∶2 adducts (BF3)2por. The close spectral correlation between the corresponding (BF3)2por and (CF3COOH)2por were suggestive of similar saddled porphyrin core structures with BF3 molecules coordinated to the two pyrrolenine nitrogen donors, and simultaneously hydrogen bonded to the pyrrole NH groups of the porphyrin macrocycle from above and below the plane of the porphyrins. The complexation of various arylpor and alkylpor with BF3 and their protonation with CF3COOH caused red shifts of the Soret bands (3 to ∼30 nm). The interaction of arylpor (except H2tmp) and also H2t(tert-Bu)p with BF3·OEt2 and CF3COOH demonstrated red shifts of the Q(0,0) bands (5.4 to 40 nm). In contrast, reactions of the alkylpor (alkyl = Me, Et, n-Pr, n-Bu) and H2tmp with BF3 or CF3COOH displayed blue shifts of the Q(0,0) bands (−13.5 to −31.8 nm). The observed differences in the Q(0,0) bands shifts for the complexation of arylpor versus alkylpor are presumably related to the relative co-planarity of the meso-aryl groups with the porphyrin core, and the possible π-interactions in the former. It is noteworthy that while the UV-vis spectrum of H4t(tert-Bu)p2+ was very sensitive to excess amounts of CF3COOH, the UV-vis spectrum of (BF3)2H2t(tert-Bu)p showed no changes in the presence of additional BF3·Et2O. The 1H and 13C NMR spectra of the 1∶2 adducts demonstrated a general correspondence with those of the related protonated porphyrins. However, the pyrrole NH signals of the (BF3)2por were upfield shifted to an unusual extent as compared to those of the diprotonated H2por2+ species. This effect presumably is due to the weaker NH hydrogen bonding of the 1∶2 molecular complexes compared to the protonated porphyrins. It was also observed that, in contrast to the gradual upfield shifts of the NH signals of H2por2+ with increasing CF3COOH concentration, the NH signals of (BF3)2por complexes remained fixed and independent of BF3·OEt2 concentration. 19F and 11B NMR spectra of various (BF3)2por showed upfield shifts of both 11B and 19F signals relative to those of BF3·OEt2. The observed larger upfield shifts of 11B (−6.48 to −6.71 ppm) signals than those of 19F (−3.53 to −4.20 ppm), apparently reflect direct coordination of the B atoms to the nitrogen donors and their closer proximity to the porphyrin core. The results of ab initio calculations illustrated that in (BF3)2H2tpp the two BF3 molecules are coordinated to the pyrrolenine nitrogen donors and are hydrogen-bonded to the pyrrole NH groups. Also calculations indicated that the addition of a BF3 molecule to the 1∶1 species, BF3H2tpp, is more favorable (2.4 kcal mol−1) than its coordination to H2tpp, and the 1∶2 molecular complex is more stable (14.5 kcal mol−1) than the 1∶1 adduct. A mechanism is proposed to explain the absence of the 1∶1 adduct and the observed symmetric NMR spectra of the pyrrole rings and fluorines in (BF3)2por.

 Please wait while we load your content...
Please wait while we load your content...