Synthesis and characterization of amino acid-functionalized hydroxyapatite nanorods
Abstract
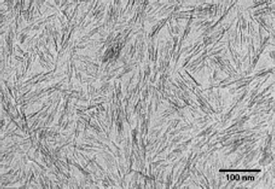
Synthesis of aqueous colloids of amino acid-functionalized hydroxyapatite (HAP; Ca10(PO4)6(OH)2) nanoparticles with rod-like morphology and uniform size was achieved by hydrothermal crystallization at 80 °C and pH 9 in highly supersaturated solutions with a Ca2+∶amino acid molar ratio of 1∶2. Stable suspensions of positively charged nanorods, less than 80 nm in length and ca. 5 nm in width, were obtained in the presence of glycine, alanine, serine, lysine and arginine, and together with FTIR spectroscopy data this was consistent with a general model in which the α-carboxylate of the amino acid was preferentially bound to the crystal surfaces. Variations in the size and shape of the nanoparticles with amino acid used were consistent with differences in the strength of binding at the HAP surfaces. In comparison, stable colloids could not be prepared with aspartic acid due to a relatively low surface charge; instead, the precipitate consisted of highly elongated nanorods, ca. 150 × 3.5 nm in dimension. XRD textural analysis suggested a model for nanorod formation based on the oriented aggregation and fusion of primary crystallite domains specifically along the c axis direction. Colloids consisting of HAP/alanine or HAP/arginine nanoparticles were cross-linked in situ by thermal, hydrothermal or chemical polymerisation of surface-attached and unbound amino acids to produce aggregated nanostructures with variable degrees of long-range order.
- This article is part of the themed collection: New developments in bio-related materials

 Please wait while we load your content...
Please wait while we load your content...